-
2015
Founded in 2015, Valgen Medtech Co., Ltd. established its headquarters in Hangzhou High-Tech Zone (Binjiang) Hangzhou and set up an R&D center in Shenzhen. The company's office and manufacturing site cover a floor space of nearly 10,000 square meters.
-
600+
Valgen Medtech specializes in the collaboration between hospitals and medical device manufacturers to provide integrated interventional treatment solutions to patients with bicuspid and tricuspid diseases. Valgen has applied for more than 600 patents worldwide for fundamental principles, core solutions, and/or key innovations of its products of which 200 of the patents have been granted.
-
280+
Valgen Medtech has employed more than 280 researchers, medical specialists, technicians, and manufacturing workers throughout China, nearly 20% of which are high-end talents with master's or doctoral degrees.
-
5+
Four of its products have passed NMPA's special review for innovative medical devices, and five of its products have been officially marketed.
Solutions
Approved by China's National Medical Products Administration in November 2023
Approved by EU in April 2025
Approved by Hong Kong(China), Indonesia,Colombia,Venezuela,Turkey, and Peru in 2025
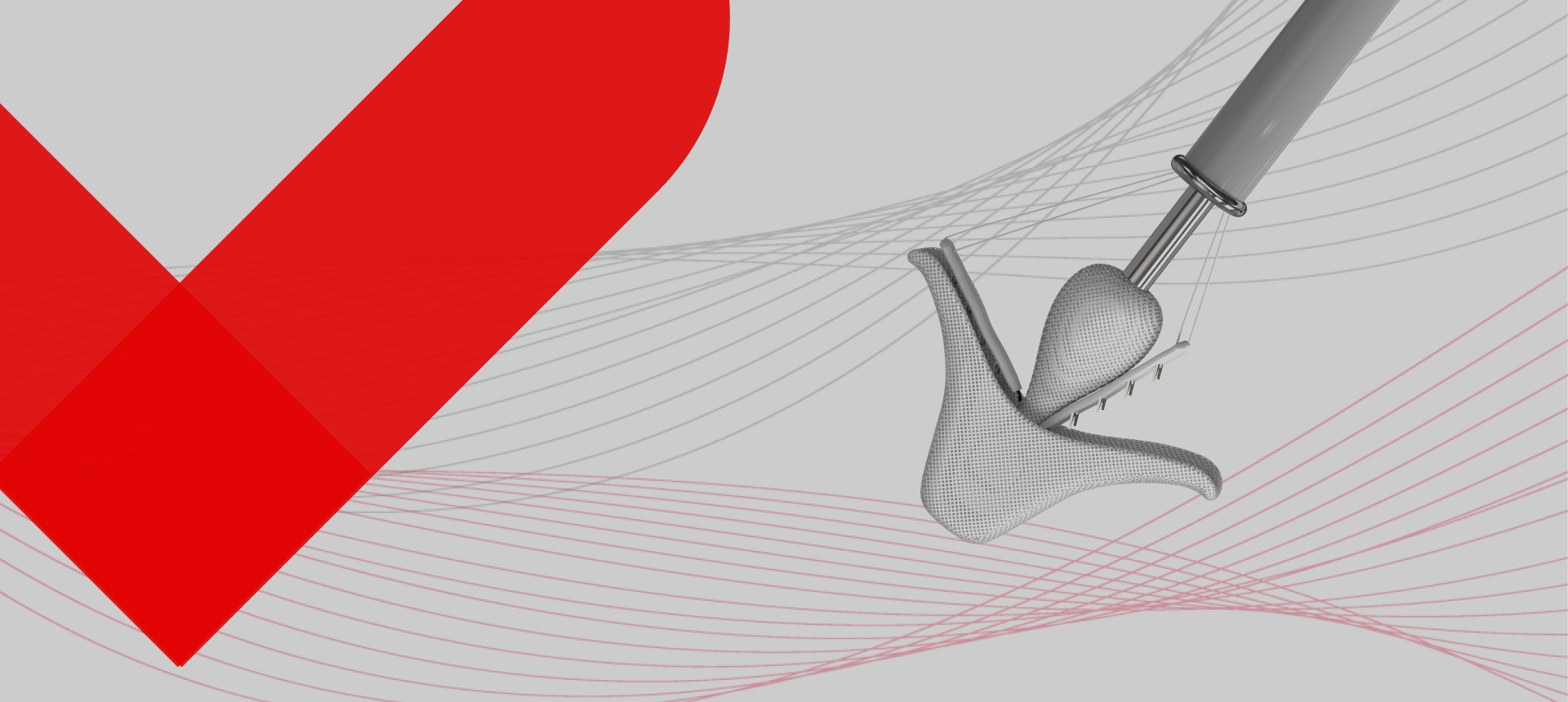
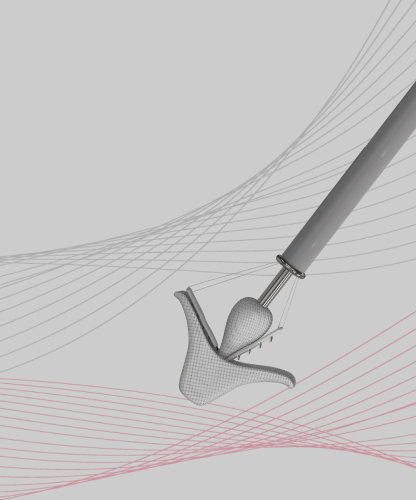
Designed for safe and accurate control.Applicable for a range of anatomies,controllable clipping for safety,Accurate and intuitive operation.
DragonFly has independent intellectual property. By September 2025, a total of more than 150 patents had been applied for in the areas of fundamental principles, core solutions, and key innovation of products, of which 43 patents were granted.
This product is intended for balloon dilatation of the cardiac aortic valve.
For creating access to the left heart via transfemoral vein transseptal puncture into the left atrium.
The product is intended for interventional diagnosis, treatment and therapeutic surgeries of the cardiovascular system (excluding the coronary arteries). It is primarily used in interventional procedures that involve guiding the insertion of instruments into blood vessels and either positioning the instruments or establishing vascular access.



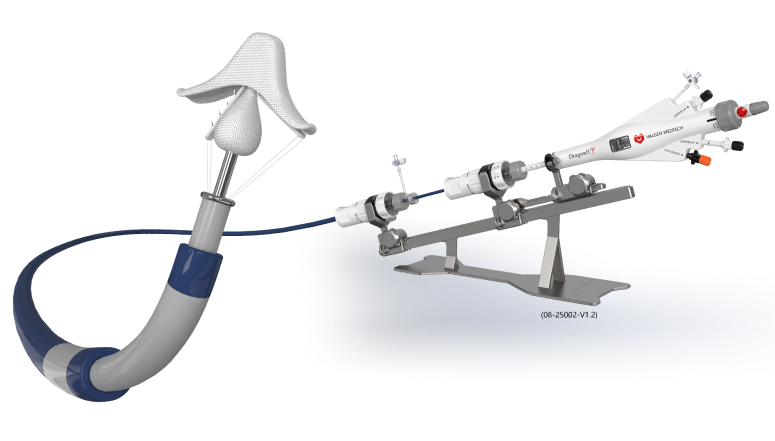
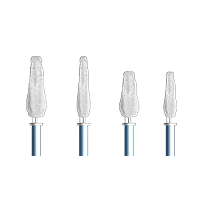
Four clip sizes

Adjustable clipping angle
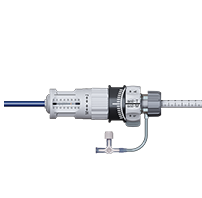
Readable indicators and rotary knobs

Independent grasping

Central filler

Unique catheter design


Innovative R&D
Abiding by the scientific philosophy of "Collaborations of Industry, Academia, Research, and Application", Valgen Medtech always gives priority to saving the lives of patients and improving the quality of life of patients in its constant exploration of the improvement of new technology and new applications and sharpening of the industry's core competitive edge to provide technology support to Chinese innovative medical devices.
-
Artificial chordae tendineae implantation system with position detection device
-
Artificial chordae tendineae implantation system with negative pressure device
-
Artificial chordae tendineae implantation system with auxiliary clipping device
-
A lockpin and interventional remote suture locking device for locking and tightening of sutures
-
Valve clipping devices and valve clipping systems
-
A heart valve prosthesis and its stent, and heart valve prosthesis replacement system
-
Absorbable suture system
-
An atrioventricular valve clipping device and atrioventricular valve clipping system
-
Zhejiang Technology-oriented Small- and Medium-sized Enterprise Certificate
Patient Care

News and Information
Providing doctors and patients with more innovative technology-based minimally-invasive interventional treatment solutions
-
2023-12-13
Breakthrough|Valgen Medtech's Transcatheter Hypertrophic Cardiomyopathy (HCM) Ablation Technique Successfully Used for Exploratory Clinical Application in 10 Patients
A team led by Profession Fang Zhenfei at the Second Xiangya Hospital of Central South University has once again applied the DragonFire myocardium RF ablation system and the transcatheter cardiomyopathy RF ablation needle and its guiding system……
Read More
-
2023-11-30
Big News|Valgen DragonFly™ Transcatheter Mitral Valve Repair System Granted Marketing Approval in China by the NMPA
November 29, 2023 - Hangzhou Valgen Medtech Co., Ltd. (Valgen Medtech) announced that its in-house developed DragonFly™ Transcatheter Mitral Valve Repair System (DragonFly™) has been granted marketing approval by the National Medical Products Administrati
Read More
-
2023-11-22
Valgen Medtech and Venus Medtech reached a consensus on signing a letter of intent for exclusive cooperation on strategic marketing to speed up the promotion of techniques for diagnosis and treatment of valvular heart disease (VHD) in China
November 22, 2023 -- Venus MedTech (Hangzhou) Inc. (Venus Medtech) and Hangzhou Valgen Medtech Co., Ltd. (Valgen Medtech) reached a consensus on signing a letter of intent for exclusive cooperation on strategic marketing for the Valgen Medtech DragonFly™
Read More