Big News|Valgen DragonFly™ Transcatheter Mitral Valve Repair System Granted Marketing Approval in China by the NMPA

November 29, 2023 - Hangzhou Valgen Medtech Co., Ltd. (Valgen Medtech) announced that its in-house developed DragonFly™ Transcatheter Mitral Valve Repair System (DragonFly™) has been granted marketing approval by the National Medical Products Administration (NMPA). The DragonFly™ is the first Chinese transfemoral valve clip product to be approved by the NMPA.

Dragonfly™ Transcatheter Mitral Valve Repair System Receives Marketing Approval
The National Medical Products Administration (NMPA) recently reviewed and approved the innovative product registration application for the Dragonfly™ Transcatheter Mitral Valve Repair System submitted by Hangzhou Valgen Medtech Co., Ltd.
The product consists of a guiding sheath and a valve clip system. The valve clip system comprises a valve clip and a delivery system. The elastic central filler structure of the valve clip can improve the tightness of clipping, reduce central residual regurgitation, and lower the clipping force of the valve leaflet; Furthermore, the valve clip has been designed with a separate grasping valve leaflet and reposition grasping functionality which helps to improve the precision of operations and lower the risk of valve clip detachment and valve leaflet perforation.
The product is suitable for degenerative mitral regurgitation (MR≥3+) patients who, in the opinion of the cardiologist team, are at high risk during surgery but have appropriate mitral valve anatomic structures. The marketing of the product will provide an additional option for clinical treatment.
Drug administration regulatory authorities will implement vigorous post-marketing surveillance to safeguard patients receiving treatment with the device.
The DragonFly™ is the result of nearly ten years of joint development by Valgen Medtech and a team of cardiologists led by Academician Wang Jian’an at the Second Affiliated Hospital of Zhejiang University School of Medicine and a team of materials scientists led by Academician Zhang Xingdong at the National Engineering Research Center for Biomaterials, Sichuan University. The development of the product was supported by National Key R&D Program for the "14th Five-Year Plan".
The first implantation of the DragonFly™ into a patient in China in 2020
initiated the era of Chinese mitral valve repair systems
Mitral regurgitation (MR) is the most common valvular disease of the heart (VDH). Severe MR accounts for up to one fourth of patients with heart failure and decreased ejection fraction. If not treated, patients with severe MR have a 3-year mortality rate of 40-50% and a 5-year heart failure hospitalization (HFH) rate of 90%. The morbidity of MR increases significantly with age. Nearly half of MR patients have high risk factors that prevent them from receiving surgical operation. The transcatheter mitral valve edge-to-edge repair procedure (TEER) was first developed in 2003. By 2022, more than 200,000 TEER procedures had been conducted.
According to Clinical Pathway for Transcathether Mitral Valve Edge-to-edge Repair in China (2022 Edition), there are 7.5 million Chinese MR patients who need interventional treatment, and the morbidity of MR increases significantly with age. The disease’s morbidity is 0.3% in people aged 35~50, 0.9% in people aged 51~64, and 2.2% in people aged ≥65. In people aged >65 who have moderate to severe VDH, the morbidity of MR is 19.1%, and more than two thirds of these patients are ineligible for surgical treatment due to being of a senile age, complications, and other risk factors; the 5-year mortality rate of these patients is 50%.
On July 23, 2020, a team of cardiac surgeons led by Academician Wang Jian’an at the Second Affiliated Hospital of Zhejiang University School of Medicine successfully completed TEER with the DragonFly™ in an elderly patient. This success initiated the treatment of MR with the transfemoral transcatheter edge-to-edge mitral valve repair system developed in house in China.
Clinical studies and devotion to development
laid down a solid foundation for the product
Since its first successful implantation in a Chinese patient in 2020, the DragonFly™ has been investigated in clinical trials in 27 sites and implanted into more than 300 patients in China so far. The clinical data shows the system has good maneuverability and long-term safety and effectiveness.
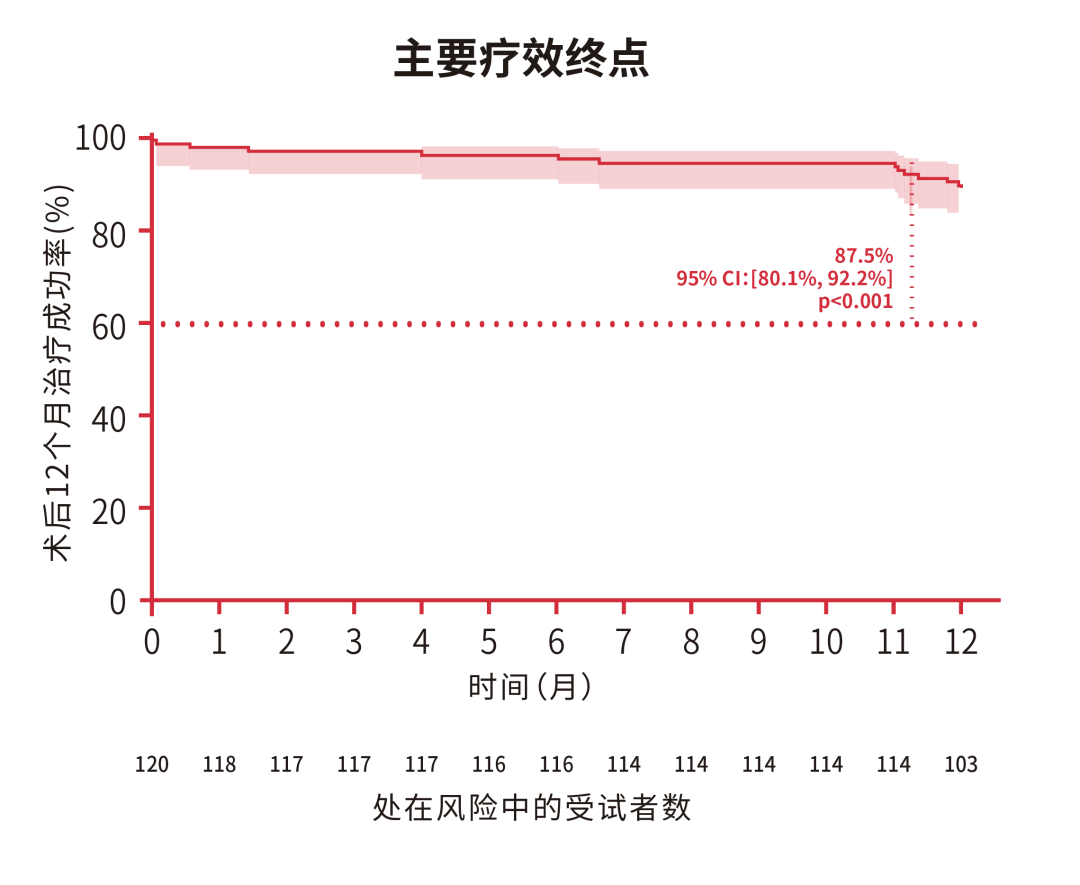
In the period from May 2021 to January 2022, the DragonFly-DMR trial in China had enrolled and treated 120 patients. According to the follow-up data, the immediate procedural success rate and device implantation success rate of the DragonFly in 120 patients were both 99.2%. With the DragonFly, the 12-month postoperative treatment success rate was 87.5% (95% CI: 80.1%, 92.2%), the results from 12 months of postoperative follow-up showed the patients’ cardiac function and quality of life had been significantly improved. The impressive treatment results in the confirmatory clinical trial indicated that the predefined primary effectiveness and safety endpoints had been successfully achieved.
During the surgical procedure, 1 valve clip was successfully implanted in 52.5% of the subjects and 2 valve clips were successfully implanted in 42.5% of the subjects in the DragonFly™ Group. The mean postoperative immediate and 12-month transmitral pressure gradients (TMPG) were 2.88±1.34mmHg, 3.19±1.38mmHg, respectively. The above performance data of the DragonFly™ device were comparable to the results of the latest clinical trials of MitraClip and PASCAL.
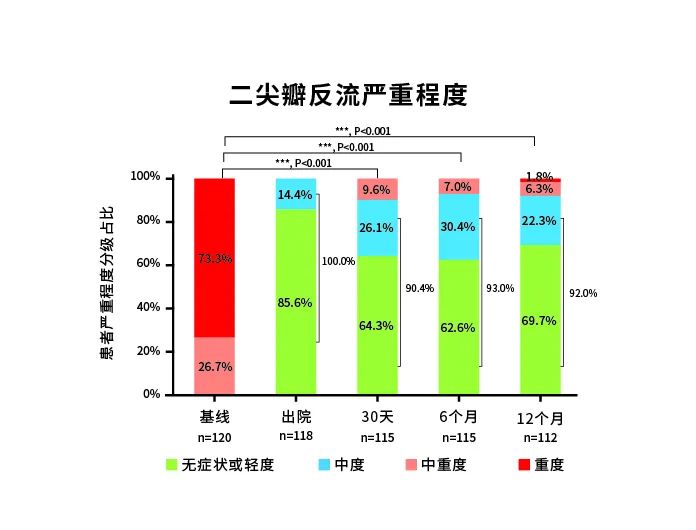
Furthermore, the 12-month postoperative follow-up results showed the patients’ cardiac function and quality of life had been significantly improved: the percentage of NYHA Class I/II subjects increased from 32.4% at baseline to 93.6% at 12 months postoperative (P<0.001); Kansas City Cardiomyopathy Questionnaire (KCCQ) score increased by 30.64±18.35 from baseline (P<0.001).
The clinical trial results of the DragonFly-DMR showed that the DragonFly™ system had good safety, effectiveness, and maneuverability for the treatment of degenerative mitral regurgitation; considering the device's cost effectiveness, it could be a good option for DMR patients who are at high risk during surgery.
Fast deployment in the field of mitral valve repair
Lead TEER enters a new stage
The key to the development of transcatheter mitral valve repair devices lies in technological innovation. So far, only two TEER products have been approved by CE/FDA: MitraClip (Abbott Vascular) and PASCAL (Edwards Lifesciences). Both are based on transfemoral catheterization of the femoral vein. A comprehensive survey of the replacement of foreign products revealed three major trends in technological innovation.
Change of the surgical approach from the transapical route to the transfemoral route for substantial reduction of invasive operations and effective decrease of post-operative adverse events. More convenient operation of devices by cardiac intervention surgeons for bringing more opportunities to more patients who are at high risk during surgery.
Reduced complications of devices and improvement of device maneuverability for clinical promotion.
The shift from single operation to combined operations using combined devices not only provides diverse solutions for patients with complicated pathological changes of the mitral valve but also improves the effectiveness and durability of the therapeutic effect.
The DragonFly™ product for the following four major innovative design features can achieve safe and effective clamping for direct and convenient operation and precise and reliable therapeutic effect across a wide variety of patients.
Mechanical clipping implemented by the combination of extendable central filler and stepless self-locking of valve clip for guaranteed stable valve leaflet grasping, reduction of valve leaflet tension and valve leaflet injury, lower central residual regurgitation, and lower postoperative transvalvular pressure gradient.
Four clip sizes for better matching of variable valve leaflet anatomic structures and more options to fit mitral regurgitation patients with a variety of pathological changes and anatomic structures.
Separate grasping function for one-sided grasping and a higher procedural success rate and operative efficiency in patients with complicated disease.
The combination of unique three-segment catheter and an adjustable accurately maneuverable delivery system for accurate and convenient visualized, articulated, and calibrated operation.
DragonFly™ has independent intellectual property rights. By September 2023, a total of more than 150 patents had been applied for in the areas of basic principles, core solutions, and key innovations of the product, including 95 Chinese and foreign patents of inventions and 28 PCT applications; 30 patents were granted. Three technical achievements were published in JACC: Asia and other renowned international journals.
Comments by Academic Wang Jian’an from the Second Affiliated Hospital of Zhejiang University School of Medicine:"The DragonFly™ Transcatheter Mitral Valve Repair System is a typical paradigm of the joint efforts of hospitals and medical device manufacturers to promote the in-house development of innovative medical devices. We successfully completed the world’s first trial of the product in animals in August 2019 and the world's first clinical application of the DragonFly™ in humans; in 2022, the enrollment of the last subject in the DMR confirmatory clinical trial was completed; during the process, we all witnessed the advancement and strength of medical devices developed in house in China, which helped to overcome the existing technological bottleneck and could result in a breakthrough occurring soon in China. In the future, we will keep improving our products to meet clinical needs and strive to provide better treatment options to MR patients worldwide."
Comments by Mr. Zhang Tingchao, General Manager of Valgen Medtech: "Everything we do, we do it for the patients, and for the good of the healthcare industry and society. Valgen Medtech will, based on its technological expertise and industry experience it has gained in the field of transcatheter mitral valve repairs, provide patients throughout the world with more cost-effective services.
The technology for the diagnosis and treatment of structural heart disease is a frontier technology of the world nowadays. The technology has been developing rapidly in China in recent years thanks to the rise and continual progress in the strength of Chinese science and technology. Today, the regulatory approval of the DragonFly™, a Chinese transfemoral valve clip system, marks yet another milestone in China’s medical device development after the approval of the first Chinese transcatheter aortic valve replacement (TAVR) device in 2017. If the diagnosis and treatment of structural heart disease attains a world-leading standard in China in the future, the approval of the DragonFly™ today will be a milestone.
Founded in 2015, Valgen Medtech Co., Ltd. is headquartered in Hangzhou High-Tech Zone (Binjiang) Hangzhou and has established an R&D center in Shenzhen. The company’s office and manufacturing site cover a floorage of nearly 10,000 square meters. The company specializes in the development of interventional treatment products and technologies for mitral valve and tricuspid valve diseases. It has been accredited as a national and provincial (Zhejiang) technology-oriented SME, a high and new technology R&D center, and engineering research center in Hangzhou. In 2022, Valgen Medtech took the lead in undertaking a project in the National Key R&D Program for the "14th Five-Year Plan" and participated in numerous national and provincial key R&D programs. The company was listed as a Chinese Unicorn Enterprises in 2020. Valgen Medtech has applied for more than 500 patents worldwide, of which, more than 150 patents were granted.
The company developed the DragonFly™ Transcatheter Mitral Valve Repair System in house, making it the first transfemoral transcatheter edge-to-edge mitral valve repair device in China. The development received funding from the National Key R&D Program for the "14th Five-Year Plan" in 2022.
As a believer of the philosophy that "technology innovation is the driving force behind development", Valgen Medtech has been abiding by the development strategy of "based in China, oriented towards the world" in its efforts to accelerate the development and commercial application of precision interventional diagnosis and treatment technology and provide innovative diagnosis and treatment solutions for valve repair to surgeons and patients.
Related Articles
-
Breakthrough|Valgen Medtech's Transcatheter Hypertrophic Cardiomyopathy (HCM) Ablation Technique Successfully Used for Exploratory Clinical Application in 10 Patients
2023-12-13 -
当西方遇见东方|浙二王建安院士团队与全球结构性心脏病治疗专家就经导管瓣膜介入治疗展开深入技术交流
2023-12-11 -
Big News|Valgen DragonFly™ Transcatheter Mitral Valve Repair System Granted Marketing Approval in China by the NMPA
2023-11-30

Follow Valgen Medtech




