Breakthrough|Valgen Medtech's Transcatheter Hypertrophic Cardiomyopathy (HCM) Ablation Technique Successfully Used for Exploratory Clinical Application in 10 Patients
Tenth Patient Enrolled in DragonFire Exploratory Clinical Trial
Changsha, China.A team led by Profession Fang Zhenfei at the Second Xiangya Hospital of Central South University has once again applied the DragonFire myocardium RF ablation system and the transcatheter cardiomyopathy RF ablation needle and its guiding system (collectively referred to as "DragonFire" hereinafter) in the treatment of an obstructive hypertrophic cardiomyopathy (oHCM) patient by intramyocardial RF ablation. This marks the successful completion of the 10th clinical implantation of DragonFire in an exploratory clinical trial.
The exploratory clinical trial of DragonFire, sponsored by Hangzhou Nuoqin Medical Equipment Co., Ltd. (Nuoqin Medical), a wholly owned subsidiary of Hangzhou Valgen Medtech Co., Ltd., and undertaken by Profession Fang Zhenfei (as the Principal Investigator) at the Second Xiangya Hospital of Central South University, saw successful completion of the first-in-human clinical application and enrollment of the first subject in the exploratory clinical trial in October 2022. Since the launch of the trial, surgical operation with DragonFire has been completed in 10 subjects so far. The system ran smoothly during the operation and the operation yielded the anticipated results in the subjects after the operation. Data from the six-month clinical follow-up showed the patients' cardiac function had been significantly improved and the system could be used for effective treatment of oHCM patients.

A group photo of Professor Fang Zhenfei and his team and Nuoqin Medical’s technician team
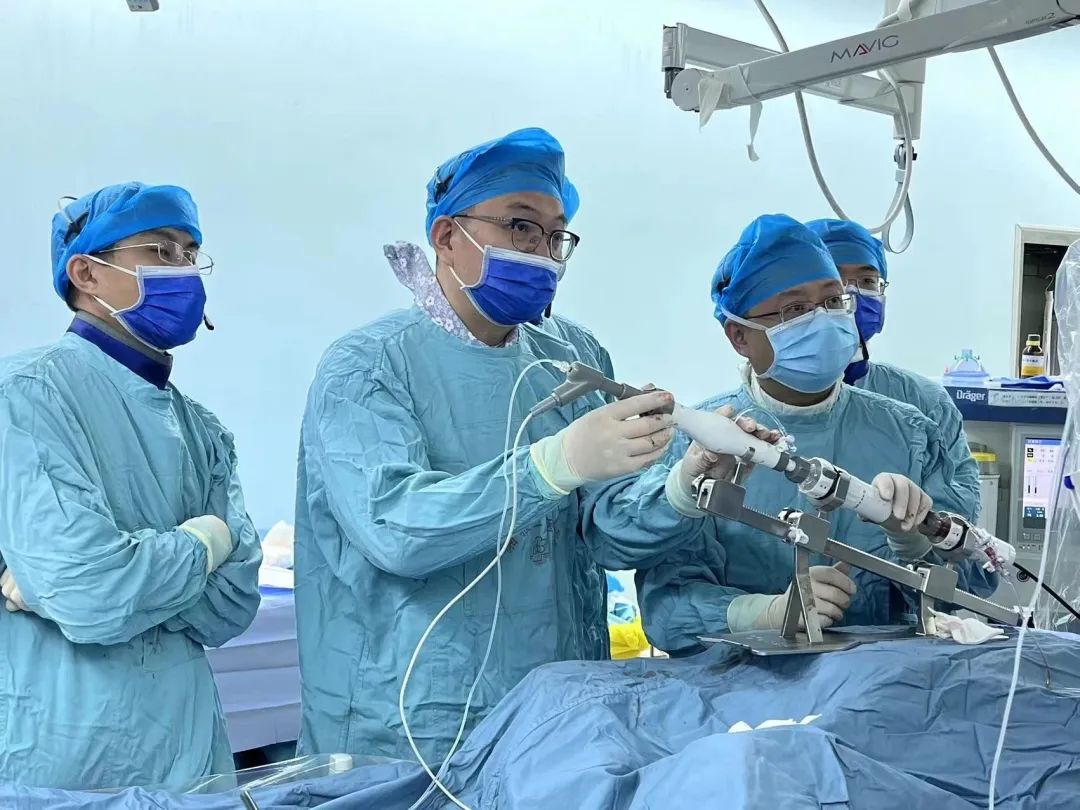
Professor Fang Zhenfei and his team performing an operation
The safety and feasibility of the new surgical method with the innovative device had been witnessed.
According to the follow-up data from the first nine patients, the mean peak left ventricular outflow tract gradient (LVOTG) values of the subjects at baseline, before discharge, 30 days, 3 months, and 6 months after the operation were: 74.78±30.47mmHg, 37.22±30.4mmHg, 25.63±14.24mmHg, 23.33±17.83mmHg, 21.00±9.00mmHg. The study results initially verified the feasibility and safety of the application of DragonFire for the treatment of oHCM. It looks like DragonFire could be a promising new solution for the treatment of HCM in China.
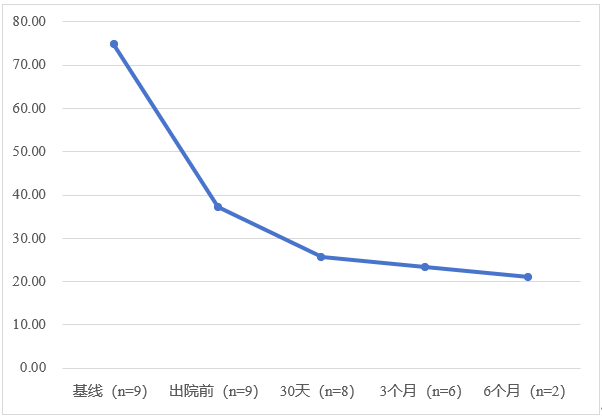
Variation of peak LVOTG values of patients at rest following surgery in the first in-man (FIM) study
The first patient treated with DragonFire in the world
The catheter was removed immediately after the operation and the patient's clinical symptoms saw a complete improvement

A group photo after the first operation on October 13, 2022 in the FIM study
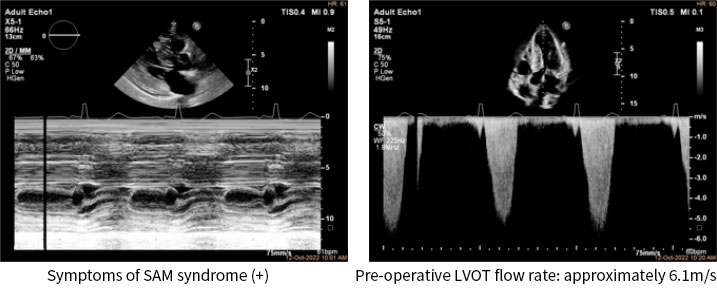
A 65 year-old female patient, who presented with such symptoms as tightness of the chest, and aggravating anhelation with nocturnal paroxysmal dyspnea, etc.; the symptoms were not been relieved following drug therapy. Echocardiography findings: LVEDD 42mm, LAD 36mm, EF 65%. Uneven significant thickening of the interventricular septum; maximum thickness 22mm; posterior wall of left ventricle thickened to 14mm; (severe) left ventricle outflow tract obstruction, symptoms of SAM syndrome (+). The symptoms were accompanied by mild mitral tricuspid regurgitation, calcification of aortic valve, and mild regurgitation. LVOT systolic flow rate increased significantly to approximately 6.1m/s, PGmax: 150mmHg.
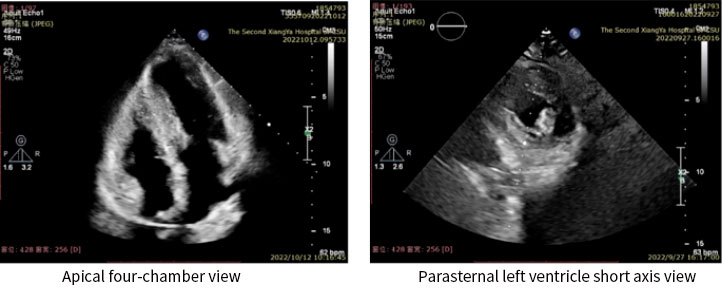
Before the operation, the technical team made use of a 3-D reconstruction technique to customize a device approach that fit the anatomical features of the patient's disease and precisely identified the area of thickening. Afterwards, a simulated ablation route was determined for the patient; a plan was made for ablation at eight sites in succession. Prior to the operation, the required ablation energy range and depth for each of the sites were estimated and a strategy for ablation from anterior septum to posterior septum was established.
During the operation, Professor Fang Zhenfei skillfully manipulated DragonFire to precisely position and puncture the thickest part of the myocardium at the basilar part of the anterior septum, intermediate septum, and the area was ablated with energy within the appropriate range.
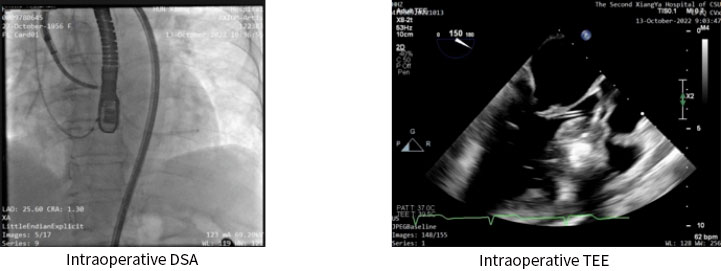
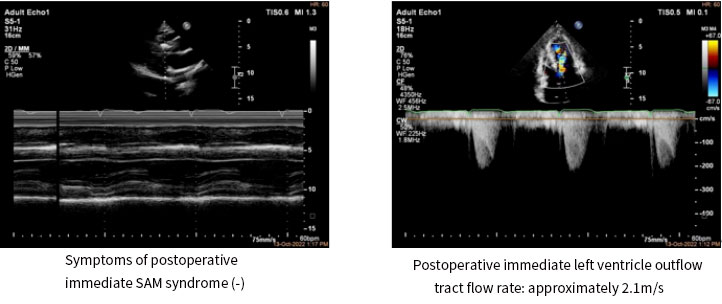
Postoperative color Doppler echocardiography suggested that the symptoms of SAM syndrome had disappeared and mitral regurgitation had improved; the LVOT systolic flow rate decreased from a pre-operative value of 6.1m/s to a post-operative value of 2.1m/s; the peak LVOTG decreased from a pre-operative value of 150mmHg to a post-operative value of 17mmHg. The immediate intraoperative ablation result was a decrease of 133mmHg in outflow tract pressure gradient.
The catheter was removed from the patient immediately after the operation and the patient's clinical symptoms significantly improved without any common post-ablation complications in oHCM patients such as arrhythmia or left ventricle low cardiac output syndrome. The patient was discharged one week after the operation.
Transcatheter transfemoral-endocardial path intramyocardial RF ablation
Building upon existing technological experience for the benefit of patients
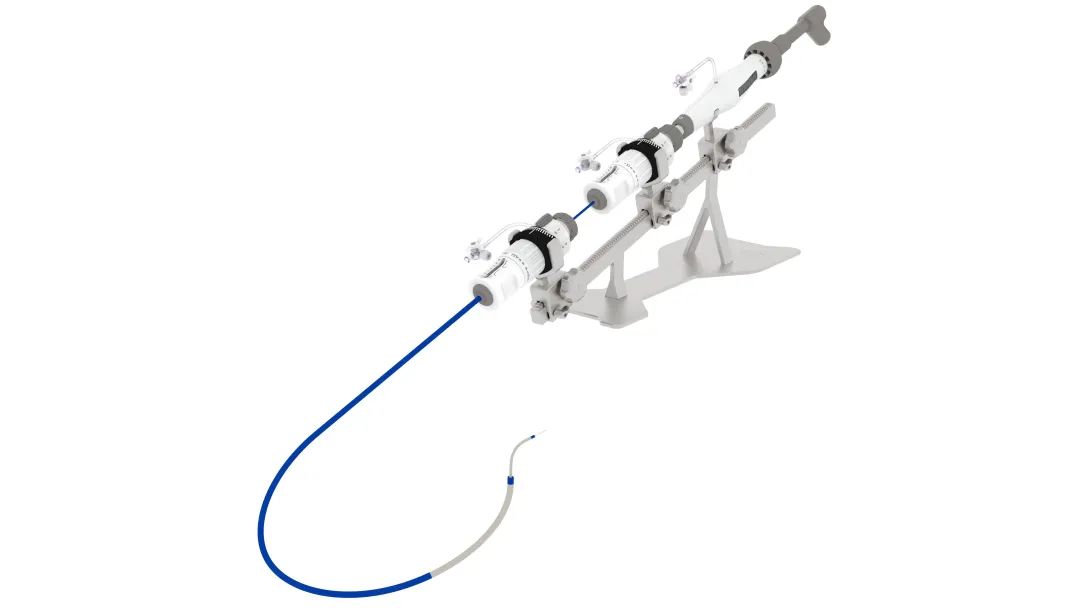
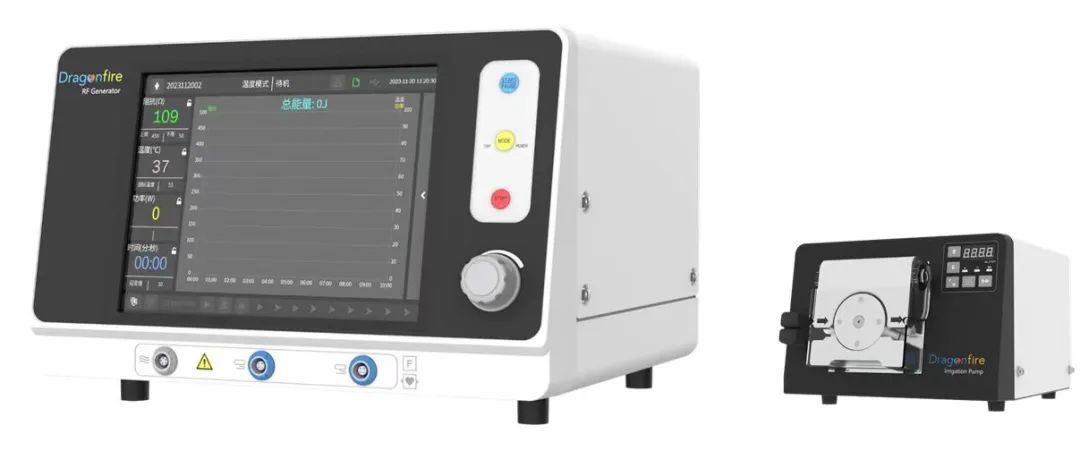
DragonFire myocardium RF ablation system and the transcatheter cardiomyopathy RF ablation needle and its guiding system
DragonFire and its innovative surgical method were developed by Nuoqin Medical and jointly investigated by Nuoqin Medical and a research team led by Professor Fang Zhenfei at the Second Xiangya Hospital of Central South University. They are for minimally-invasive intervention by transfemoral and transendocardial insertion of an ablation needle into the interventricular septum for ablation of the hypertrophic myocardium to reduce the thickness of the myocardium of the ventricular septum and improve LVOT obstruction. This ablation technique is capable of accurately predicting the ablation range and is free of the common serious complications of conventional absolute alcohol ablation or surgical operation, including outflow of injury, damage to conductive bundle, and/or post-operative low cardiac output syndrome.
DragonFire has numerous technological advantages, including no need for thoracotomy, fast therapeutic effect, less complications, small wounds, and precise and controllable ablation range. Valgen Medtech gained much relevant experience in the selection of patients, preparation of surgical strategies, surgical methods, and system operation in the early stages. Currently, a multi-center registration clinical trial is about to launch in China. It is hoped that this innovative technology will benefit patients as soon as possible.
Hangzhou Valgen Medtech Co., Ltd. (Valgen Medtech) is dedicated to the interventional treatment of structural heart diseases. Founded in 2015 in Hangzhou, Valgen Medtech applied for more than 500 patents worldwide by the end of 2023, of which more than 150 patents were granted. Three independently-developed products have passed the "Special Review of Innovative Medical Devices". Its multiple interventional therapy devices, the Dragon series, will greatly enrich clinical options in structural heart disease treatment.
The DragonFly™ Transcatheter Mitral Valve Repair System, as the first transfemoral vein edge-to-edge repair product developed in house in China, passed the review in accordance with the Special Review Procedure for Innovative Medical Devices in March 2021. The product was granted marketing approval in China by the NMPA on November 29, 2023. The development of the product was supported by the National Key R&D Program for the "14th Five-Year Plan". In addition to the DragonFly™, Valgen Medtech's marketed products include the Pu Jie Jie™ Medical Radiation Protective Shield, Magpie™ Balloon Dilatation Catheter, DragonPath™ Transseptal Puncture System, and Firework™ Stiff Guidewires.
Valgen Medtech is headquartered in Hangzhou High-Tech Zone (Binjiang) Hangzhou and established an R&D center in Shenzhen. It has been accredited as a national and provincial (Zhejiang) technology-oriented SME, a high and new technology R&D center, and engineering research center in Hangzhou. Valgen adheres to the corporate strategy of being based in China oriented towards the world, further accelerating the development and clinical application of innovative treatment technology that addresses unmet clinical needs and continuously providing better solutions for patients around the world.
Related Articles
-
Breakthrough|Valgen Medtech's Transcatheter Hypertrophic Cardiomyopathy (HCM) Ablation Technique Successfully Used for Exploratory Clinical Application in 10 Patients
2023-12-13 -
当西方遇见东方|浙二王建安院士团队与全球结构性心脏病治疗专家就经导管瓣膜介入治疗展开深入技术交流
2023-12-11 -
Big News|Valgen DragonFly™ Transcatheter Mitral Valve Repair System Granted Marketing Approval in China by the NMPA
2023-11-30

Follow Valgen Medtech




