DragonFly
Product Features
Controllable clipping for safety
i.Central filler to reduce central residual regurgitation
ii.Adjust any clipping angle within 45° for the appropriate postoperative pressure gradient
Applicable for a range of anatomies
i. Four clip sizes offer more options to the anatomy complexity
ii. Independent grasping improves the success rate of grasping
Accurate and intuitive operation
i.Readable indicators and rotary knobs increase operating accuracy and intuition
ii. Three-stage separative catheter design for accurate positioning
DragonFly Transcatheter Mitral Valve Repair System
Transfemoral transcatheter edge-to-edge mitral valve repair system

Magpie™
High rated burst pressure:
Non-compliance balloon,focusing on Expansion Force,and dilate stenosis uniformly.
Rapid Inflation and Deflation:
Larger inflation area, reduced duration of hypoperfusion and rapid pacing,better protecying cardiac function.
Eight-wing folded Design:
More organized initial state and smaller OD.The wings are formed stably and coiled tightly in the same direction for easier reuse.
Reinforced Inner Tube:
The reinforced inner tube ensures a stronger tube,and a safer use in acute arches and transverse cardiac cases.
Magpie™ Valvuloplasty Balloon Dilatation Catheter
This product is intended for balloon dilatation of the cardiac aortic valve.
Learn More

DragonPath™
Puncture sheath: Increased hardness, greater supporting force; Better anti-bending performance, sheathing canal less susceptible to deformation; Greater torsional strength, better axial orientation; Smaller frictional force, better vascular passability;
Puncture needle: For its stronger puncture strength, appropriately sharp point design, and unique post-treatment process for removing sharp edges and burrs, the needle enables comfortable control when puncturing and prevents sliding and mispuncturing.
DragonPath™ Atrial Septal Puncture System
Intended for atrial septal puncture to establish access from the right atrium to the left atrium.
Learn More
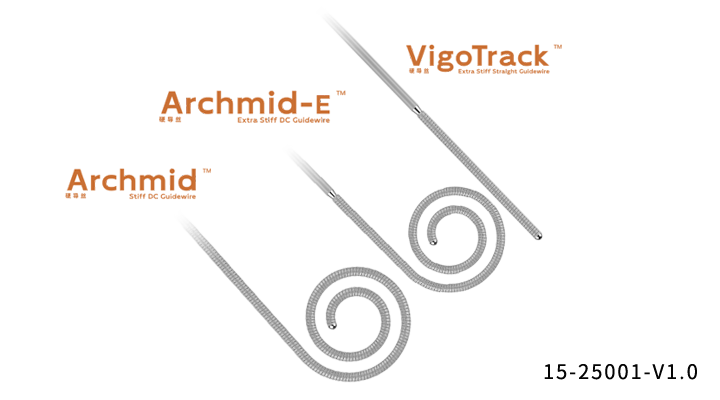
Stiff Guidewires
Consisting of core wire and wire coil.
The product surface is coated with a layer of polytetrafluoroethylene (Teflon).
The core wire and wire coil are made of 304 stainless steel.
Special double curve pre-shaped tip for easy and stable positioning in the left ventricle.
Stable morphology maintenance capacity for minimizing heart tissue injury.
Three curve sizes available for hearts of different sizes.
Stiff Guidewires
The product is intended for interventional diagnosis, treatment and therapeutic surgeries of the cardiovascular system (excluding the coronary arteries). It is primarily used in interventional procedures that involve guiding the insertion of instruments into blood vessels and either positioning the instruments or establishing vascular access.
Learn More
Related News
-
2023-11-30
Big News|Valgen DragonFly™ Transcatheter Mitral Valve Repair System Granted Marketing Approval in China by the NMPA
November 29, 2023 - Hangzhou Valgen Medtech Co., Ltd. (Valgen Medtech) announced that its in-house developed DragonFly™ Transcatheter Mitral Valve Repair System (DragonFly™) has been granted marketing approval by the National Medical Products Administrati
Read More
-
2023-10-25
TCT 2023 | 德晋医疗DragonFly™,洞见二尖瓣修复治疗新趋势
第35届经导管心血管会议(TCT.2023)在美国旧金山拉开帷幕,会议主旨为介入心脏病专家和业内专业人士分享改善患者治疗质量的最新技术成果,浙江大学医学院附属第二医院王建安教授团队再次受邀以《DragonFly™ for Mitral Transcatheter Edge-to-Edge Repair in China》作专题演讲。
Read More